HER2+ Breast Cancer
Overview and Staging
Accepting a HER2+ breast cancer diagnosis begins with understanding it. Many people are surprised to know that all breast cancers are not the same. Breast cancer is a complex disease characterized by mutations in genes and proteins that cause cells to grow out of control. Learning about why HER2+ breast cancer behaves the way it does will help you and your medical team move forward more confidently.
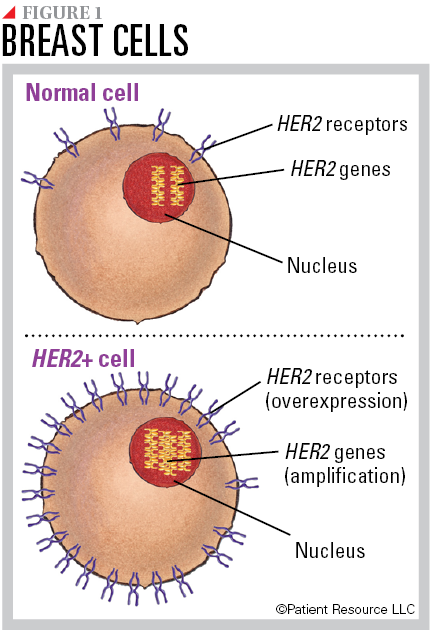
All breast cells contain two copies of the human epidermal growth factor receptor-2 (HER2) gene. The normal gene makes HER2 proteins, which are receptors on the surface of a cell. Together, the genes and protein receptors help manage how a breast cell grows, divides and repairs itself. Some breast cancers have more than the normal two copies of the HER2 gene, known as HER2 amplification, which leads to an overexpression of HER2 receptors (see Figure 1). This is called HER2 positive (HER2+) breast cancer. HER2 gene amplification and receptor overexpression can lead to cells growing out of control and developing into cancer.
A definitive HER2+ breast cancer diagnosis requires genomic testing. HER2 testing is typically performed for both invasive lobular and invasive ductal breast cancers. It is not routinely tested with ductal carcinoma in situ. Testing may be repeated if the breast cancer spreads or recurs after treatment. Approximately 20 percent of all breast cancers make extra copies of the HER2 gene.
Your breast cancer diagnosis, however, involves more than just testing for HER2. A variety of tests, including imaging and a biopsy, help determine the type of breast cancer (non-invasive or invasive) and the status of estrogen receptor (ER) and progesterone receptor (PR), which may also be drivers of the cancer.
Genomic Testing
Genomic testing is used to understand your cancer for diagnosis, staging and treatment purposes. Examining a cancer’s genes may reveal mutations that could indicate the cancer’s behavior, how aggressive it might be and how likely it is to metastasize (spread). It is not performed for every person or cancer type. In cases where it has a clinical benefit, such as with breast cancer, it may be used for the following:
- Diagnosing and staging
- Determining prognosis (outlook)
- Evaluating whether therapies are available to treat mutations that are present
- Choosing treatment and monitoring treatment effectiveness
- Watching for progression or recurrence (Tests provide a score that may be useful in determining whether hormone therapy or chemotherapy is recommended to prevent a recurrence.)
- Predicting how the tumor might behave, such as how aggressive it is and how likely it is to spread
Genomic testing is typically performed on the initial tumor biopsy material to detect biomarkers, which are substances such as genes or molecules that can be measured on the tumor itself or, at times, in the blood, plasma, urine, cerebrospinal fluid or other body fluids or tissues. Biomarkers are produced by cancer cells or other cells of the body in response to cancer. Testing may be repeated if the cancer recurs.
ER, PR and HER2 are considered the three main biomarkers in breast cancer. If the cancer is ER+ or PR+, it is driven by hormones and is referred to as hormone receptor positive (HR+). As a result, hormone (endocrine) therapy designed to block the hormones or the receptors that feed the cancer may be used as treatment.
Two tests may be performed on biopsied tumor tissue to detect the HER2 biomarker:
- Immunohistochemistry (IHC) testing measures the amount of HER2 proteins on the surface of breast cancer cells. Based on the number of proteins, a score is given to determine whether the cancer is HER2 positive (HER2+). The test uses a scale of 0 to 3+, with 0 meaning the cancer is HER2 negative (HER2-) and 3+ meaning the cancer is HER2+. If the score is 2, another test such as in situ hybridization (ISH) may be used, or you may send your results to another cancer center for a second opinion. The IHC test is typically performed first because the results can be returned quicker, and a 0 or 3+ usually requires no further testing.
- In situ hybridization (ISH) counts the number of copies of the HER2 gene. It is performed when the results of the IHC testing are inconclusive, in doubt, or an intermediate or 2+. The results take longer to return than for IHC testing, but this test is considered more definitive.
Other biomarkers in breast cancer that may be tested to monitor treatment effectiveness include cancer antigen 15-3 (CA 15-3), cancer antigen 27.29 (CA 27.29) and/or carcinoembryonic antigen (CEA). Doctors may also test for mutations in the BRAF, EGFR, KIT and PIK3CA genes, which may accompany a HER2+ diagnosis. In some cases, testing may be done for circulating tumor cells, which may indicate spread.
Understanding your Stage
One of the most common questions you may be asked when discussing your cancer diagnosis is about the stage, so it is important to know that it is much more than just a label of Stage I, II, III or IV. Staging gives your doctor deep insight into your cancer, including the extent of the disease, where it is located and whether it has metastasized (spread) to nearby organs, tissues or lymph nodes or to other parts of your body. In turn, this helps guide treatment and signals prognosis.
All types of breast cancer, including HER2+ breast cancer, are classified according to the tumor, node and metastasis (TNM) system developed by the American Joint Committee on Cancer (AJCC). Once classified, they are given a stage (see Tables 1 and 2).
The T classification categories are the same for both clinical and pathologic staging and provide information on the size and extent of the tumor within the breast.
Clinical T (described as cT) refers to the tumor size estimate based on physical/clinical examination and breast imaging; pathologic T (described as pT) refers to the size of the tumor when it has been removed and measured in the pathology laboratory.
Clinical staging for the N category (cN) describes the location and bulkiness of lymph nodes (usually in the axilla, under the arm) that seem to be malignant (from spread of the breast cancer) upon physical examination. Location and extent of any cancerous lymph nodes provide clues regarding the likelihood that the breast cancer might have spread to other organs. The pathologic N category (pN) is determined postoperatively and describes how many lymph nodes are involved.
The M category indicates whether the cancer has metastasized, or spread, to another part of the body beyond the breast and nearby lymph nodes.
In addition, your doctor will consider many other important factors before assigning your final stage: tumor grade; biomarkers, including your HER2, ER and PR status; molecular and genetic changes in cancer tissue; and results from multi-gene panels such as MammaPrint, Oncotype DX, PAM 50 (Prosigna), EndoPredict and Breast Cancer Index. These genomic tests help determine the final prognostic stage.
This information is documented on your pathology report. You can request a copy from your doctor at any time. It is helpful to have when you seek a second opinion from a doctor or cancer center with expertise in breast cancer treatment. Getting another opinion from an expert is always encouraged to ensure you are aware of all of your treatment options, including clinical trials.
How HER2+ Affects Treatment
The breast cancer cells in HER2+ breast cancer are a unique subtype of breast cancer that is less common than other types. Although they grow and spread more aggressively than other types, there are very effective treatments that target the HER2 cells. Therefore, HER2+ breast cancer requires a more targeted treatment approach in the form of anti-HER2 drug therapy.
Anti-HER2 targeted therapy is designed to slow or stop the growth of the cancer. Anti-HER2 drugs are approved for all stages of HER2+ breast cancer, and they are often used along with other treatments.
During treatment you will likely try more than one targeted therapy. It is common to begin one and then be switched to a different one. This happens because HER2+ breast cancer is characterized by multiple mutations. As a result, one of those mutations may make the cancer become resistant, or stop responding, to treatment. In that situation, the cancer will begin to grow again, requiring a change of treatment. In HER2+ breast cancer, this has been reported with some types of chemotherapy and targeted therapy. Researchers are investigating the causes of resistance with a goal of preventing it.
Table 1 - Stages of Breast Cancer
| Stage | T | N | M |
| 0 | Tis | N0 | M0 |
| IA | T1 | N0 | M0 |
| IB | T0 or T1 | N1mi | M0 |
| IIA |
T0 or T1
T2 |
N1
N0 |
M0
M0 |
| IIB |
T2
T3 |
N1
N0 |
M0
M0 |
| IIIA |
T0-T3
T3 |
N2
N1 |
M0
M0 |
| IIIB | T4 | N0-N2 | M0 |
| IIIC | Any T | N3 | M0 |
| IV | Any T | Any N | M1 |
Table 2 - AJCC TNM System for Classifying Breast Cancer
| Category | Definition |
| Tumor (T) | |
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. |
| Tis (DCIS) | Ductal carcinoma in situ. |
| Tis (Paget) | Paget disease of the nipple NOT associated with invasive carcinoma and/or carcinoma in situ (DCIS) in the underlying breast parenchyma (tissue). |
| T1 | Tumor ≤ (not more than) 20 mm in greatest dimension. |
| T1mi | Tumor ≤ (not more than) 1 mm in greatest dimension. |
| T1a | Tumor > (more than) 1 mm but ≤ (not more than) 5 mm in greatest dimension. |
| T1b | Tumor > (more than) 5 mm but ≤ (not more than) 10 mm in greatest dimension. |
| T1c | Tumor > (more than) 10 mm but ≤ (not more than) 20 mm in greatest dimension. |
| T2 | Tumor > (more than) 20 mm but ≤ (not more than) 50 mm in greatest dimension. |
| T3 | Tumor > (more than) 50 mm in greatest dimension. |
| T4 | Tumor of any size with direct extension to the chest wall and/or to the skin (ulceration or macroscopic nodules). |
| T4a | Extension to the chest wall. |
| T4b | Ulceration and/or ipsilateral (on the same side) macroscopic satellite nodules and/or edema (including peau d’orange) of the skin that does not meet the criteria for inflammatory carcinoma. |
| T4c | Both T4a and T4b are present. |
| T4d | Inflammatory carcinoma. |
| Node (N) | |
| pNX | Regional lymph nodes cannot be assessed. |
| pN0 | No regional lymph node metastasis identified or ITCs (isolated tumor cells) only. |
| pN0(i+) | ITCs (isolated tumor cells) only (malignant cell clusters no larger than 0.2 mm) in regional lymph node(s). |
| pN0(mol+) | Positive molecular findings by reverse transcriptase polymerase chain reaction (RT-PCR); no ITCs (isolated tumor cells) detected. |
| pN1 | Micrometastases; or metastases in 1-3 axillary (armpit) lymph nodes; and/or clinically negative internal mammary nodes with micrometastases or macrometastases by sentinel lymph node biopsy. |
| pN1mi | Micrometastases (approximately 200 cells, larger than 0.2 mm, but none larger than 2.0 mm). |
| pN1a | Metastases in 1-3 axillary (armpit) lymph nodes, at least one metastasis larger than 2.0 mm. |
| pN1b | Metastases in ipsilateral (on the same side) internal mammary sentinel nodes, excluding ITCs (isolated tumor cells). |
| pN1c | pN1a and pN1b combined. |
| pN2 | Metastases in 4-9 axillary (armpit) lymph nodes; or positive ipsilateral (on the same side) internal mammary lymph nodes by imaging in the absence of axillary lymph node metastases. |
| pN2a | Metastases in 4-9 axillary (armpit) lymph nodes (at least one tumor deposit larger than 2.0 mm). |
| pN2b | Metastases in clinically detected internal mammary lymph nodes with or without microscopic confirmation; with pathologically negative axillary (armpit) nodes. |
| pN3 |
Metastases in 10 or more axillary (armpit) lymph nodes;
or in infraclavicular (below the clavicle) (Level III axillary) lymph nodes; or positive ipsilateral (on the same side) internal mammary lymph nodes by imaging in the presence of one or more positive Level I, II axillary lymph nodes; or in more than three axillary lymph nodes and micrometastases or macrometastases by sentinel lymph node biopsy in clinically negative ipsilateral internal mammary lymph nodes; or in ipsilateral supraclavicular (above the clavicle) lymph nodes. |
| pN3a |
Metastases in 10 or more axillary (armpit) lymph nodes (at least one tumor deposit larger than 2.0 mm);
or metastases to the infraclavicular (below the clavicle) (Level III axillary) lymph nodes. |
| pN3b | pN1a or pN2a in the presence of cN2b (positive internal mammary nodes by imaging);
or pN2a in the presence of pN1b. |
| pN3c | Metastases in ipsilateral (on the same side) supraclavicular (above the clavicle) lymph nodes. |
| Note: (sn) and (f) suffixes should be added to the N category to denote confirmation of metastasis by sentinel node biopsy or FNA/core needle biopsy respectively, with NO further resection of nodes. | |
| Metastasis (M) | |
| M0 | No clinical or radiographic evidence of distant metastases. |
| cM0(i+) | No clinical or radiographic evidence of distant metastases in the presence of tumor cells or deposits no larger than 0.2 mm detected microscopically or by molecular techniques in circulating blood, bone marrow, or other nonregional nodal tissue in a patient without symptoms or signs of metastases. |
| cM1 | Distant metastases detected by clinical and radiographic means. |
| pM1 | Any histologically proven metastases in distant organs; or if in non-regional nodes, metastases greater than 0.2 mm. |



