Sarcoma
Clinical Trials
Today’s clinical trials are tomorrow’s standard of care treatments, and they are a treatment option worthy of serious consideration for anyone who has cancer — and especially for those diagnosed with a rare cancer.
Hundreds of sarcoma clinical trials are currently taking place across the country to evaluate different aspects of sarcoma treatment, from new drug therapies to tests for identifying biomarkers. As a direct result of clinical trials such as these over the years, people are enjoying longer, better quality lives.
Each clinical trial is carefully designed and planned, then conducted according to rigorous standards to ensure consistent and safe treatment for every patient. Institutional Review Boards, the Data and Safety Monitoring Board and the FDA dictate strict safety measures to protect people who participate in clinical trials. Each study also has eligibility requirements, such as the subtype and stage of sarcoma, conditions specific to what the trial is studying, treatment history, age, overall health and other criteria.
It’s important to know that if you choose to take part in a clinical trial, you will always receive the equivalent of the current standard of care treatment for your type and stage of cancer.
Benefits and Risks
Participating in a sarcoma clinical trial offers many potential benefits.
- Allows you access to leading-edge treatments that aren’t yet commercially available.
- Provides an alternative if your current treatment no longer works as well as before or if the sarcoma is so rare that no standard treatment exists.
- Ensures even closer monitoring than usual by medical professionals as they gather required data on your progress.
- Gives you the opportunity to contribute to medical science by helping researchers find new and better treatments for future sarcoma patients.
Clinical trials may also pose potential risks and inconveniences. Nearly all cancer treatments have side effects, and therapies studied in clinical trials are no different. It’s also possible the treatment won’t be more effective than the current standard. In addition, clinical trials have a prescribed schedule that could conflict with yours, so before you commit, check your calendar to make sure you’ll be available during the stated timeframe.
Understanding Informed Consent
Choosing to participate in a sarcoma clinical trial is a significant decision. A process called Informed Consent helps you make sure the clinical trial you’re considering is the right fit.
Once you confirm your interest with your doctor, you’ll get detailed instructions and an Informed Consent form. The form will outline the clinical trial’s purpose, how it will work, safeguards in place, potential benefits and risks, possible side effects, how you’ll be monitored, number of appointments, patient rights and privacy information and more. The form should also state the treatment that is the current standard of care for your subtype and stage of sarcoma.
Take time to review the form thoroughly and discuss your questions or concerns with your doctor. Before you sign, contact your insurance provider(s) to find out which procedures, tests and appointments are covered and which (if any) you’ll be expected to pay.
Once you’ve weighed the benefits and risks and have decided the clinical trial is right for you, sign and return the Informed Consent form. Understand that signing the form doesn’t “lock you in” to the clinical trial. Participating in a clinical trial is voluntary. You have the right to withdraw from the study at any time and for any reason. Just let your doctor know your decision to switch to standard of care.
Consent Differs for Children
Parents must give consent for a child younger than 18 to participate in a clinical trial. However, it is important for children to be included in the process so they can better understand their treatment plan. The research will be explained in age-appropriate language using videos or other visuals (see Pediatric Sarcoma).
How to Search for a Sarcoma Clinical Trial
In addition to talking with your doctor about clinical trials, you can search for them on your own. Many online resources are available, but navigating through thousands of clinical trials may be overwhelming and confusing. Step-by-step instructions below will help guide you.
Before you begin, have your exact diagnosis, pathology report and a list of your previous treatments handy. If you don't find a clinical trial right away, keep checking because new trials are frequently added. You may choose to continue searching while you move ahead with your current treatment plan. If you're interested in a clinical trial that no longer accepts participants, your doctor may be able to appeal to the U.S. Food and Drug Administration (FDA) for expanded access know as "compassionate use".
[STEP 1] FILL IN YOUR INFORMATION
Enter Your Diagnosis
For example, enter “sarcoma.” To get more results, do additional searches such as "pediatric sarcoma", "soft tissue sarcoma", "Ewing sarcoma", "advanced sarcoma", etc., and compare results.
Desired Location
If you prefer a clinical trial close to home, enter your address. If you have the ability to travel for treatment, enter additional locations.
Other Terms
Refine your search by adding a treatment type such as "targeted therapy", or a specific drug name. You may also add a Clinical Trial Identifier, which is an eight-digit code (preceded by “NCT”) assigned to each trial.
[STEP 2] READ YOUR SEARCH RESULTS
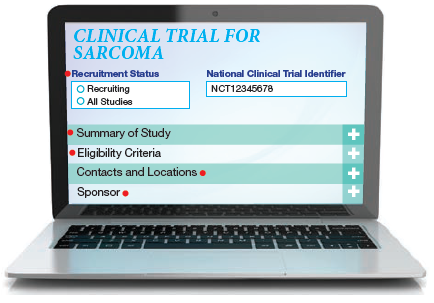
Recruitment Status
This indicates whether the trial is actively seeking patients, not yet recruiting or otherwise inactive. The status will change, so check for updates.
Summary of Study
Here you’ll find details about the purpose of the clinical trial and the treatment being studied. This section may be difficult to interpret because it's usually written for a medical provider. If that's the case, print the information to discuss with your doctor.
Eligibility Criteria
This outlines the criteria you must meet to be eligible for the trial, such as the stage of disease, previous treatments you have had, sites of metastasis, and your age and overall health.
Contacts and Locations
This may include contact information for clinical trial investigators, staff or sponsors, who may be able to offer more details about the study.
Sponsor
This is the organization responsible for the clinical trial. It may be a pharmaceutical company, university, biotechnology company of the National Cancer Institute.



