HER2- Advanced Breast Cancer
HER2- Advanced Breast Cancer roadmap
Being told you have advanced or metastatic breast cancer has likely come as a shock. Start with learning about the type of breast cancer you have. Understanding more about it will help you begin to make a plan for moving forward alongside your multidisciplinary health care team. Although a cure is not yet available for metastatic breast cancer, research advances are making it possible for more people with this diagnosis to live longer, higher-quality lives.
Advanced vs. Metastatic
Advanced breast cancer is a diagnosis that applies to locally advanced and/or metastatic disease. It may be detected upon diagnosis (referred to as de novo). It may also be found in a follow-up exam after treatment for early-stage breast cancer or because of new symptoms.
- Locally advanced describes breast cancer that is in the breast and/or axilla (underarm) that can be seen on imaging. This is typically Stage III disease and, if no obvious or overt metastatic disease is detected on body imaging such as CT, PET or bone scans, it is treated with curative intent.
- Metastatic breast cancer describes malignant (cancerous) cells that began in breast tissue but have broken away and traveled through the bloodstream or lymph vessels to one or more distant sites in the body, and the disease involvement in other organs is visible on body imaging such as CT, PET or bone scans. When this occurs, the cancer is staged as or upgraded to Stage IV.
Multiple subtypes of breast cancer have been identified, and researchers continue to find differences in the ways these breast cancers grow and respond to treatment. These are some of the types of breast cancer that have the potential for metastasizing:
- Invasive ductal carcinoma, the most common, starts in the lining of the milk ducts in the breast when abnormal cells grow out of control, forming a mass that spreads from the ducts to normal breast tissue.
- Invasive lobular carcinoma, the second most common, starts in the lobules (glands that make milk) and spreads to surrounding normal tissue.
- Inflammatory breast cancer (IBC) is a rare, very aggressive form that grows rapidly and is automatically classified as Stage III or Stage IV, depending on whether it has spread. IBC tends to spread quickly, making it challenging to treat. Most cases are invasive ductal carcinomas in which cancer cells block the lymph vessels, causing the lymph fluid to build up. This results in breast tenderness, swelling, redness and pain. Breast skin can thicken and appear pitted like an orange peel.
Your experience with a metastatic diagnosis will be different than before if you were previously diagnosed at an earlier stage when there was an end date to active treatment. The goal of treating metastatic breast cancer is to slow the cancer’s growth, or stop its progression, for as long as possible while keeping side effects manageable.
Staging and Classification
The tumor, node and metastasis (TNM) system developed by the American Joint Committee on Cancer (AJCC) is used to stage and classify breast cancer (see Tables 1 and 2). The system includes the tumor (T) size, cancer cells found in nearby lymph nodes (N) and cancer that has metastasized (M), or spread, to other parts of the body, such as the bones, brain, liver or lungs.
Patients with advanced breast cancer generally need a biopsy of the metastatic site(s) to confirm the diagnosis as breast cancer and not as a second different type of cancer, and to assess biomarkers on the breast cancer that has spread to distant sites. A second primary breast cancer is not metastatic breast cancer and usually represents a new breast cancer requiring repeat diagnostic work-up (including biomarker testing) followed by treatment.
Table 1. Stages of Advanced Breast Cancer
| Classification | Definition |
| IIIA |
T0-T3, N2, M0
T3, N1, M0 |
| IIIB | T4, N0-N2, M0 |
| IIIC | Any T, N3, M0 |
| IV | Any T, Any N, M1 |
Table 2. AJCC TNM System for Classifying Breast Cancer
| Category | Definition |
| Tumor (T) | |
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. |
| Tis (DCIS) | Ductal carcinoma in situ. |
| Tis (Paget) | Paget disease of the nipple NOT associated with invasive carcinoma and/or carcinoma in situ (DCIS) in the underlying breast parenchyma (tissue). |
| T1 | Tumor ≤ (not more than) 20 mm in greatest dimension. |
| T1mi | Tumor ≤ (not more than) 1 mm in greatest dimension. |
| T1a | Tumor > (more than) 1 mm but ≤ (not more than) 5 mm in greatest dimension. |
| T1b | Tumor > (more than) 5 mm but ≤ (not more than) 10 mm in greatest dimension. |
| T1c | Tumor > (more than) 10 mm but ≤ (not more than) 20 mm in greatest dimension. |
| T2 | Tumor > (more than) 20 mm but ≤ (not more than) 50 mm in greatest dimension. |
| T3 | Tumor > (more than) 50 mm in greatest dimension. |
| T4 | Tumor of any size with direct extension to the chest wall and/or to the skin (ulceration or macroscopic nodules). |
| T4a | Extension to the chest wall. |
| T4b | Ulceration and/or ipsilateral (on the same side) macroscopic satellite nodules and/or edema (including peau d’orange) of the skin that does not meet the criteria for inflammatory carcinoma. |
| T4c | Both T4a and T4b are present. |
| T4d | Inflammatory carcinoma. |
| Node (N) | |
| pNX | Regional lymph nodes cannot be assessed. |
| pN0 | No regional lymph node metastasis identified or ITCs (isolated tumor cells) only. |
| pN0(i+) | ITCs (isolated tumor cells) only (malignant cell clusters no larger than 0.2 mm) in regional lymph node(s). |
| pN0(mol+) | Positive molecular findings by reverse transcriptase polymerase chain reaction (RT-PCR); no ITCs (isolated tumor cells) detected. |
| pN1 | Micrometastases; or metastases in 1-3 axillary (armpit) lymph nodes; and/or clinically negative internal mammary nodes with micrometastases or macrometastases by sentinel lymph node biopsy. |
| pN1mi | Micrometastases (approximately 200 cells, larger than 0.2 mm, but none larger than 2.0 mm). |
| pN1a | Metastases in 1-3 axillary (armpit) lymph nodes, at least one metastasis larger than 2.0 mm. |
| pN1b | Metastases in ipsilateral (on the same side) internal mammary sentinel nodes, excluding ITCs (isolated tumor cells). |
| pN1c | pN1a and pN1b combined. |
| pN2 | Metastases in 4-9 axillary (armpit) lymph nodes; or positive ipsilateral (on the same side) internal mammary lymph nodes by imaging in the absence of axillary lymph node metastases. |
| pN2a | Metastases in 4-9 axillary (armpit) lymph nodes (at least one tumor deposit larger than 2.0 mm). |
| pN2b | Metastases in clinically detected internal mammary lymph nodes with or without microscopic confirmation; with pathologically negative axillary (armpit) nodes. |
| pN3 |
Metastases in 10 or more axillary (armpit) lymph nodes;
or in infraclavicular (below the clavicle) (Level III axillary) lymph nodes; or positive ipsilateral (on the same side) internal mammary lymph nodes by imaging in the presence of one or more positive Level I, II axillary lymph nodes; or in more than three axillary lymph nodes and micrometastases or macrometastases by sentinel lymph node biopsy in clinically negative ipsilateral internal mammary lymph nodes; or in ipsilateral supraclavicular (above the clavicle) lymph nodes. |
| pN3a |
Metastases in 10 or more axillary (armpit) lymph nodes (at least one tumor deposit larger than 2.0 mm);
or metastases to the infraclavicular (below the clavicle) (Level III axillary) lymph nodes. |
| pN3b | pN1a or pN2a in the presence of cN2b (positive internal mammary nodes by imaging);
or pN2a in the presence of pN1b. |
| pN3c | Metastases in ipsilateral (on the same side) supraclavicular (above the clavicle) lymph nodes. |
| Note: (sn) and (f) suffixes should be added to the N category to denote confirmation of metastasis by sentinel node biopsy or FNA/core needle biopsy respectively, with NO further resection of nodes. | |
| Metastasis (M) | |
| M0 | No clinical or radiographic evidence of distant metastases. |
| cM0(i+) | No clinical or radiographic evidence of distant metastases in the presence of tumor cells or deposits no larger than 0.2 mm detected microscopically or by molecular techniques in circulating blood, bone marrow, or other nonregional nodal tissue in a patient without symptoms or signs of metastases. |
| cM1 | Distant metastases detected by clinical and radiographic means. |
| pM1 | Any histologically proven metastases in distant organs; or if in non-regional nodes, metastases greater than 0.2 mm. |
Illustrated Stages of Advanced Breast Cancer
Understanding Biomarkers
Determining your biomarker status is a key part of diagnosing breast cancer. Biomarkers are substances such as genes or molecules that can be measured in the blood, plasma, urine, cerebrospinal fluid or other body fluids or tissues with genomic testing. They are produced by cancer cells or other cells of the body in response to cancer. Genomic testing is a laboratory test used to detect biomarkers on the initial tumor biopsy material.
The most common biomarkers in breast cancer are the hormone receptors estrogen (ER) and progesterone (PR), as well as the genes and receptors for human epidermal growth factor receptor-2 (HER2). Every person has ER, PR and HER2 receptors, but the levels of each can change on the cancer cells. Each can be positive (ER+, PR+ and HER2+) or negative (ER-, PR- and HER2-), or they may occur in various combinations. These biomarkers are tested for during the initial diagnostic process on a biopsy sample.
Hormone receptor positive (HR+) breast cancers indicate that both estrogen and progesterone are supporting the cancer’s growth. If it is hormone receptor negative (HR-), these hormones are not driving the cancer. It is also possible that the cancer is being driven by only one of the hormones, such as ER+/PR- or ER-/PR+.
Normal breast cells contain two copies of the HER2 gene, which makes HER2 proteins that are receptors on the surface of a cell. The HER2 genes and protein receptors help manage how a breast cell grows, divides and repairs itself. When a breast cell has more than two copies of the HER2 gene or more receptors than normal, it is considered HER2 positive (HER2+) breast cancer (flip this guide over to read more about HER2+ breast cancer). If the breast cell has no or very few HER2 receptors, it is considered HER2 negative (HER2-) or HER2-low.
To determine the cancer’s HER2 status, a tissue biopsy sample will be examined in a laboratory. The two tests that may be used are immunohistochemistry (IHC), which looks for biomarkers, and in situ hybridization (ISH), which looks at genes or chromosomes in a cell.
IHC is typically performed first. It measures the amount of HER2 proteins on the surface of breast cancer cells. A score is given to determine the HER2 status, which is measured with a scale of 0 to 3+.
- A score of 0 or 1+ means the cancer is HER2-.
- A score of 1+ or 2+ without HER2 gene amplification is considered borderline and ISH testing may be performed, which counts the number of copies of the HER2 gene, or you may send your results to another cancer center for a second opinion. If the ISH test is negative, the cancer is considered to be HER2-low.
- A score of 3+ means the cancer is HER2+.
This guide focuses on advanced and metastatic HER2-, HER2-low and triple negative breast cancer (TNBC).
Understanding HER2-, HER2-Low and TNBC
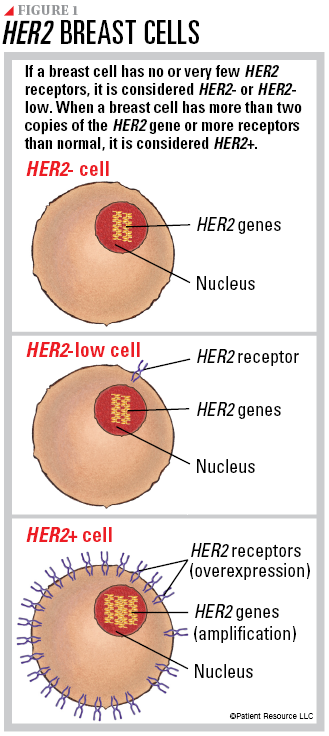
As you begin learning about the type of breast cancer you have, it may help to understand the differences between HER2-, the new HER2-low designation and TNBC, which is ER-, PR- and HER2-, meaning none of these are fueling the cancer’s growth and none are detectable on the cancer cell. All of these breast cancers may be diagnosed at any stage.
Hormone receptor (HR) status for people with HER2- or HER2-low may be either HR+ or HR-. Hormone (endocrine) therapy is only used to treat cancers with either estrogen or progesterone receptor expression regardless of HER2 status.
HER2- breast cancer occurs when the cancer cells do not have HER2 protein receptors on their surface, which is indicated by an IHC score of 0 or 1+ (see Figure 1). This indicates that the cancer is not being driven by HER2 and the receptors are not detected or are only minutely present. If the IHC test is definitive, ISH testing may not be necessary.
Cancer cells that are HER2- may grow more slowly and are less likely to recur (return) or spread to other parts of the body than cancer cells with a large amount of HER2 receptors on their surface (HER2+). This subtype does not respond to anti-HER2 therapy but may be treated with other drug therapies.
HER2-low breast cancer is a new classification of breast cancer. It may have an IHC score of 1+ or 2+ without HER2 gene amplification. A score of 1+ or 2+ means there is a low level of HER2 receptors on the surface of the cancer cells, but not enough to be considered HER2+. Because the score falls between HER2- and HER2+, additional testing is required to confirm this type. As a result, your biopsy sample will also have ISH testing.
This new classification is important because patients who are HER2- traditionally have not benefitted from anti-HER2 targeted therapies, which are used to treat HER2+ breast cancers. New research, however, has shown that if a breast cancer cell has even a few HER2 receptors on its surface, the patient may benefit from some anti-HER2 therapies.
With this HER2-low classification, many people who previously did not have access to the therapies that target HER2 may now qualify for them and benefit in the metastatic setting.
TNBC is diagnosed when the tumor cells do not have estrogen receptors (ER-), progesterone receptors (PR-) or large amounts of HER2 protein receptors (HER2-) on their surface. This means that the cancer is not being driven by hormones or HER2. This type will have an IHC score of 0 to 1+.
Because TNBC is not driven by hormones or HER2, it has fewer treatment options. As a result, chemotherapy is often used and may be combined with other drug therapies, including immunotherapies. TNBC diagnoses that were HER2- may be retested to determine whether they are actually HER2-low, which could make targeted therapy an option. Your biopsy sample may also be tested for the PD-L1 protein to determine whether you are a good candidate for immunotherapy.
Clinical trials are ongoing and continue to look for new ways to treat TNBC. Ask your doctor if you should consider joining a clinical trial.
Ongoing Monitoring
Ongoing testing will become a very important part of your life when you have advanced or metastatic breast cancer. Your doctors will watch you closely to learn about any symptoms you have, detect new signs of cancer growth, check for treatment resistance (when cancer no longer responds to a certain treatment) and identify other changes in your health.
Periodically, your doctor may order a biopsy to determine whether the biomarkers in the cancer have changed (mutated), which may alter future treatment options. This will include retesting for any changes in your ER, PR and HER2 status. In some cases, a HER2+ diagnosis can turn into HER2-, which would change your treatment plan.
Circulating tumor cells (CTCs), which are cells from the tumor that have broken off and are moving throughout the bloodstream, may also be measured to monitor whether the cancer is growing or has become resistant to any therapies in your treatment. Higher numbers of CTCs may indicate the cancer is growing.
To manage the anxiety that accompanies continual follow-up visits and testing, find out when to expect results, how you will receive them, and who will deliver them to you.
Questions to ask your doctor
What is the difference between HER2-, HER2-low and TNBC?
What treatments are available for my type of breast cancer?
Could I be HER2-low?
How confident are you in the test results, or is additional testing needed?
If I was HER2- at diagnosis, could I be retested to determine whether I might be HER2-low?
Are HR and HER2 the same thing?



