Cancer Immunotherapy
Exploring Immunotherapy
Immunotherapy is revolutionizing how doctors treat certain types of cancer. Sometimes referred to as biologic therapy or biotherapy, immunotherapy harnesses the potential of the body’s own immune system to fight cancer.
This content offers an easy-to-understand explanation of immunotherapy and explores several types that are approved to treat cancer. Other novel treatments that are not yet FDA-approved for these and other cancers may be available through clinical trials as researchers continue to improve existing therapies and explore new ones. Additional strategies, such as using certain drugs for the treatment of some solid tumors that are microsatellite instability-high cancer (MSI-H), may also be considered.
Immunotherapy 101
Immunotherapy can be a difficult concept to understand. The science of it essentially changes a body’s immune system so that it will recognize an illness that it may not have been able to fight before. Once the body can recognize the illness, it will fight it. Imagine how a fish tank’s environment works.

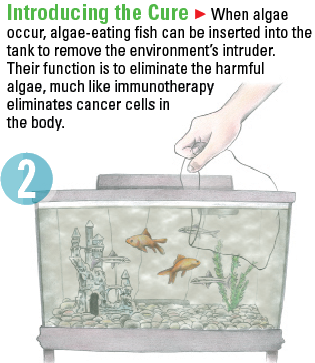

Training the immune system to respond to cancer has the potential for a more lasting response that can extend beyond the end of treatment, making it very different from the following types of cancer treatments.
- Surgery is the removal of the tumor and surrounding normal tissue.
- Chemotherapy involves drugs to stop the growth of cancer cells.
- Radiation therapy uses high-energy X-rays or other types of radiation to kill cancer cells or stop them from growing.
- Targeted therapy includes drugs or other substances that attack cancer cells directly, usually by targeting a specific abnormal gene or protein.
- Stem cell transplantation takes blood stem cells harvested from you or a donor and infuses them back into your body after high-dose chemotherapy.
- Clinical trials are research studies that investigate potential cancer treatments.
Some of these treatments, such as targeted therapy, chemotherapy and radiation therapy, may have primary effects on the tumor cell in addition to influencing the immune response. A type of targeted therapy, for example, looks for antigens on B-cells, and B-cells are part of the immune system. Research is just beginning to understand how certain agents influence immunity and how they may be used to optimize treatment by engaging the patient’s immune system in the fight against cancer.
Some drug therapies, including immunotherapy, may be prescribed as brand names, generics or a new form called biosimilars.
Immunotherapy is less likely to affect healthy tissues and cells, which may reduce the likelihood and severity of side effects in general. Serious reactions called immune-related adverse events (IRAEs), however, are possible. Your doctor will let you know what to watch for. If you begin to experience any IRAEs or other side effects, contact your doctor at once (see Side Effects).
To be a candidate for immunotherapy, you must meet certain criteria, such as having a functioning immune system, not having an autoimmune disorder and not taking immunosuppressive medications.
Biomarker testing may also be required because some immunotherapies are approved to treat cancers in people with specific biomarkers present. Biomarkers, also known as tumor markers, molecular markers, biological markers or serum markers, are substances, such as genes, proteins or molecules that are produced by cancer cells or other cells of the body in response to cancer. They can be measured in the blood, plasma, urine, cerebro-spinal fluid or other body fluids or tissues. Not all patients respond to immunotherapy, and doctors are now using biomarker testing to determine who is most likely to respond.
A prognostic biomarker provides information about a person’s overall cancer outcome, regardless of therapy. A predictive biomarker helps doctors guide and monitor a specific treatment approach. Research is ongoing to find more predictive and prognostic biomarkers and to better determine which patients may respond to immunotherapy.
Adoptive Cellular Therapy (T-cell therapy)
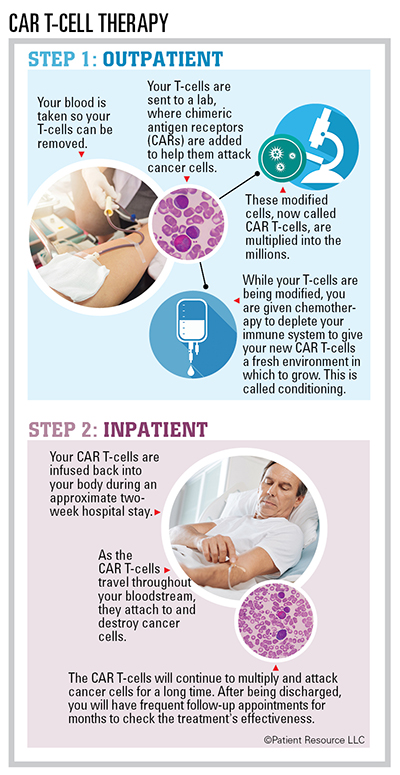
Adoptive cellular therapy is a treatment that enhances or changes the body’s own immune cells to be able to fight cancer. There are two main strategies. In one strategy, the doctor isolates T-cells that have attached to a patient’s tumor (tumor-infiltrating lymphocytes, or TILs), helps them multiply outside of the body, and then administers them back to the patient.
In the second strategy, a patient’s T-cells are collected from a blood sample, and new receptors called chimeric antigen receptors (CARs) are added that enable the T-cells to recognize specific antigens (foreign substances such as bacteria, viruses or parasites) on cancer cells. These engineered T-cells are called CAR T-cells. They are multiplied in a laboratory and then infused back into the patient. The goal is for the T-cells to multiply, seek and destroy the cancer cells that carry those specific antigens.
The first two CAR T-cell therapies were recently approved to treat certain blood cancers. This breakthrough therapy is giving hope to people with B-cell lymphoma and leukemia. Clinical trials are evaluating its application to other blood cancers, including lymphomas, leukemias and multiple myeloma. The hope is that CAR T-cell therapy will be an alternative to chemotherapy and stem cell transplantation as first-line treatment for many, if not all, blood cancers.
The benefits of CAR T-cell therapy include a high response rate, the possibility of long-term remission, the need for only one infusion in most cases, and the long-term effectiveness of CAR T-cells, which are designed to work for many years in your bloodstream. Drawbacks include the high cost of the treatment and the risk of dangerous side effects, such as cytokine-release syndrome, neurologic toxicities, B-cell aplasia, tumor lysis syndrome or anaphylaxis. Most side effects are reversible, but they should be taken seriously.
Immune Checkpoint Inhibitors
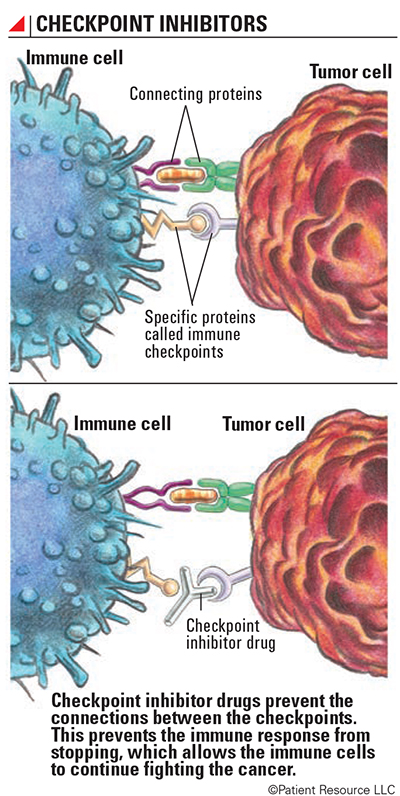
A primary function of the immune system is to determine which cells or substances are self or non-self. The immune system only makes enough white blood cells to fight non-self antigens present in the body. After an attack, the immune system must slow down. It does this through the use of checkpoints.
Checkpoints keep the immune system “in check,” preventing an attack on normal cells by using regulatory T-cells (see Explaining the Immune System). When the correct proteins and cell receptors connect, a series of signals is sent to the immune system to slow down once an immune response is finished. Three checkpoint receptors that slow down the immune system have been identified for their roles in cancer treatment.
- CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) is a receptor that binds with certain molecules to tell the immune system to slow down.
- PD-1 (programmed cell death protein 1) is a receptor involved with telling T-cells to die and to reduce the death of regulatory T-cells (suppressor T-cells). Both slow down an immune response. PD-1 can tell the immune system to slow down only if it connects with PD-L1.
- PD-L1 (programmed death-ligand 1) is a protein that, when combined with PD-1, sends a signal to reduce the production of T-cells and enable more T-cells to die.
When PD-1 (the receptor) and PD-L1 (the protein) combine, the reaction signals it’s time to slow down. CTLA-4, however, can connect with more than one protein, which is a more complex reaction than with PD-1 and PD-L1. When CTLA-4 combines with any of the various proteins, it also tells the immune system to slow down.
One of the ways cancer cells can outsmart the immune system is by producing PD-L1 and using it as camouflage so that T-cells will see them as normal cells. T-cells expect only normal cells to produce PD-L1, so when a T-cell encounters PD-L1 on a cancer cell, it is tricked into signaling the immune system to slow down. This is how cancer can hide from the immune system.
Checkpoint inhibitor drugs prevent connections between checkpoints. This prevents the immune response from slowing down, which allows the immune cells to continue fighting the cancer. When an immune checkpoint inhibitor is given, it’s as if the immune system develops X-ray vision and can see through the cancer cell’s camouflage. This keeps the immune response from slowing down and also helps the immune system recognize cancer cells as foreign cells.
The following immune checkpoint inhibitors are currently approved as cancer treatments.
- Anti-CTLA-4 antibodies allow T-cells to continue fighting cancer cells instead of shutting down.
- Anti-PD-1 drugs allow for the continued or increased production of T-cells and enable them to continue fighting cancer.
- Anti-PD-L1 molecules allow T-cells to see through some tumor cells’ disguises, recognize them as the enemy and attack them.
Monoclonal Antibodies
Antibodies (a type of protein) are the body’s way of tagging a specific antigen (foreign substance). They bind to the antigen, which allows the rest of the immune system to recognize the antigen as foreign and target it for destruction.
Monoclonal antibodies (mAbs) are laboratory-made antibodies that are designed to target specific tumor antigens. They can work in different ways, such as flagging targeted cancer cells for destruction, blocking growth signals and receptors and delivering other therapeutic agents directly to targeted cancer cells. They can also be created to carry cancer drugs, radiation particles or laboratory-made cytokines (proteins that enable cells to send messages to each other) directly to cancer cells. When a mAb is combined with a toxin, such as a chemotherapy drug, it travels through the system until it reaches the targeted cancer cell. Then it attaches to the surface, gets swallowed by the tumor cell and breaks down inside the cell, releasing the toxin and causing cell death. Combining mAbs with radiation particles, a treatment known as radioimmunotherapy, allows for radiation to be delivered in lower doses over a longer period of time. This direct form of radiation delivery typically damages only the targeted cells.
Different types of mAbs are used in cancer treatment, but they should not be confused with monoclonal antibodies that directly attack certain components in or on cancer cells, a type of treatment known as targeted therapy.
- Naked mAbs work by themselves. No drugs or radioactive particles are attached.
- Conjugated mAbs have a chemotherapy drug or a radioactive particle attached to them. They are used to deliver treatment to the cancer cells. These also are referred to as tagged, labeled or loaded antibodies.
- Bispecific mAbs are made up of two different mAbs and can attach to two different proteins at the same time. In some cases, the two proteins may both be on a cancer cell. In other cases, one protein may be on a cancer cell and one on a T-cell, thereby connecting the T-cell to a cancer cell.
Nonspecific Immune Stimulation
This treatment strategy boosts the whole immune system instead of just specific parts. It can be used alone or in combination with other treatments to produce increased and longer-lasting immune responses. Different types of nonspecific immune stimulation include the following.
Cytokine immunotherapy aids in immune cell communication and plays a big role in the full activation of an immune response. This type of immunotherapy works by introducing large amounts of the following laboratory-made cytokines to the immune system to promote specific immune responses.
- Interleukins help regulate the activation of certain immune cells.
- Interferons boost the ability of certain immune cells to attack cancer cells.
- Granulocyte-macrophage colony stimulating factors (GM-CSFs) stimulate the bone marrow, promoting the growth of immune and blood cells and the development of dendritic cells, which become antigen-presenting cells (cells that show the antigens to T-cells).
Modified bacteria have been changed to ensure they will not cause the disease to spread while stimulating an immune response in certain cancers.
Toll-like receptor agonists recognize patterns in bacteria or viruses and produce a signal that activates the immune cell to attack. The immune system often detects germs through a series of toll-like receptors found on the surface of, or inside, most immune cells.
Oncolytic Virus Immunotherapy
An oncolytic virus only attacks and kills cancer cells. This type of immunotherapy uses viruses that directly infect tumor cells to cause an immune response against the infected cells. The only oncolytic virus currently approved uses a weakened version of the herpes simplex virus. It has been changed from the original and contains the GM-CSF cytokine. The virus targets specific cancer cells, infects them and duplicates itself continuously within the cell until it ruptures. This rupture kills the cell and releases the GM-CSF cytokine produced by the virus to promote an overall immune boost. This process increases the chance that the attack can also begin killing cancer cells that have not been infected with the virus. Other viruses are being evaluated as potential cancer treatments.
Vaccinations
Two types of vaccines are used against cancer: preventive vaccines and treatment vaccines. Preventive vaccines are given before a person develops cancer with the goal of stopping it from forming. Currently, preventive vaccinations are available for human papillomavirus (HPV) and for hepatitis B virus (HBV).
Treatment vaccines treat existing cancers. These vaccines are created from either viruses or tumor cells that have been changed in a laboratory. These vaccines direct immune cells to the cancer cells. Some of these vaccines are custom-made for the patient’s specific tumor type while others are “off-the-shelf” vaccines that contain one to more than 100 antigens common to the patient’s type of cancer.
Additional types of cancer vaccinations include the following.
Tumor cell vaccines are made from tumor cells similar to a patient’s cancer type. (In rare cases, these vaccines are made from a patient’s own tumor.) In some cases, the tumor cells are changed in the laboratory to express a new property or are treated with drugs that make the tumor cells or their components easier for the immune system to recognize. The vaccines are treated with radiation to prevent spreading and are then injected back into the body to help the immune system recognize remaining cancer cells.
Antigen vaccines are typically made from one to five of the antigens that are either unique to or overexpressed (more than needed) by tumor cells. They may be specific to a certain type of cancer but are not patient-specific.
Dendritic cell vaccines are made from white blood cells removed from the patient. The cells are sent to a laboratory and changed into dendritic cells. When they’re injected back into the patient, they share the antigen information with the T-cells so the cells releasing that specific antigen are targeted and destroyed.
Vector-based vaccines are made from altered viruses, bacteria, yeast or other structures that can be used to get antigens into the body. Often, these germs have been altered so that they no longer cause disease. Some vaccines can be used to deliver more than one cancer antigen at a time. Vector-based vaccines are injected into the body to create an immune response, both specific and overall. Tumor-specific vectors are changed to train the immune system to recognize, target and destroy cancer cells.
The only approved treatment vaccine is a dendritic cell vaccine that contains the prostatic acid phosphatase protein that targets prostate cancer cells. Other treatment vaccines are experimental but may be available through participation in a clinical trial.



